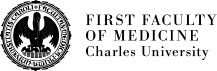Hope for Rare Anemia Patients: Czech Scientists Create "Perfect" Mouse Model to Test New Drugs
Kokavec, J., Turková, T., Schuster, B., Prochazka, J., Spoutil, F., Jamrichová, K., Holečková, M., Chalupský, K., Beck, I.M., Ruberte, J., Vale, M., Čermák, L., Stopka, T. and Sedlacek, R. (2026), Rps19R67∆ mutation creates a model of Diamond–Blackfan anemia and reveals downstream mediators of p53 pathway. HemaSphere, 10: e70302. (IF 14.6, D1) https://doi.org/10.1002/hem3.70302
More information can be found HERE.



![]()
Leukemia cells escape therapy by reprogramming oxidative metabolism
A new paper from our Laboratory of Stem Cells Proteomics is exploring novel aspects of hypomethylating therapy with azacitidine, its impact on the redox proteome of leukemia cells, and how resetting redox homeostasis relates to azacitidine resistance.
Press release available HERE.
Read the full paper HERE.
Cena kolegia děkana za excelentní publikaci 2024
2. místo
Ujevic A, Knizkova D, Synackova A, Pribikova M, Trivic T, Dalinskaya A, Drobek A, Niederlova V, Paprckova D, De Guia R, Kasparek P, Prochazka J, Labaj J, Fedosieieva O, Roeck BF, Mihola O, Trachtulec Z, Sedlacek R, Stepanek O, Draber P. TBK1-associated adapters TANK and AZI2 protect mice against TNF-induced cell death and severe autoinflammatory diseases. Nat Commun. 2024 Nov 19;15(1):10013. doi: 10.1038/s41467-024-54399-4. [PubMed]
3. místo
Kupcova K, Senavova J, Jura F, Herman V, Rajmonova A, Pacheco-Blanco M, Chrbolkova T, Hamova I, Davis RE,
Havranek O. Vertical targeting of the PI3K/AKT pathway at
multiple points is synergistic and effective for non-Hodgkin lymphoma.
Exp Hematol Oncol. 2024 Nov 1;13(1):108. doi:
10.1186/s40164-024-00568-6. [PubMed]


Novartis workshop 2025
It was a great honor for us to take part in organizing a joint workshop that brought together experts from the First Faculty of Medicine of Charles University – BIOCEV and Novartis Czech Republic. This collaboration allowed us to create an inspiring environment for open discussions on product life-cycle management and drug safety. Participants gained not only valuable insights but also an opportunity to exchange experience across the fields of medicine, pharmacy, and drug development.
I highly appreciate the excellent professional level of all speakers as well as the active engagement of the audience. I believe that this event will serve as an inspiration for further joint projects and bring long-term benefits to the entire professional community.
Milan Jakubek, Head of Department BIOCEV, 1.LF UK



Cena Grantové agentury ČR pro Petera Drábera
Peter Dráber získal cenu předsedy Grantové agentury ČR v kategorii lékařské a biologické vědy!
GAČR vyznamenání uděluje za mimořádné výsledky dosažené při řešení grantových projektů ukončených v předchozím roce.
Rozhovor pro magazín Univerzity Karlovy - Forum, naleznete zde.
Peter Dráber a jeho tým se ve výzkumu na 1. LF UK v BIOCEVU zaměřil na receptor pro interleukin 17, který hraje zásadní roli v rozvoji některých autoimunitních onemocnění. Odhalili velmi důležitou a dosud neznámou součást tohoto receptoru. To může přinést nové možnosti pro cílené ovlivňování rozvoje určitých zánětlivých onemocnění, jako je například lupénka.

Gratulujeme!

Science Outreach Matters
On Saturday, September 13, scientists from the BIOCEV, First Faculty of Medicine, Charles University joined the “Get to Know Dolnobřežansko” festival in Vestec.
Our goal was to present the work of our researchers to the public in a fun way and perhaps even inspire the next generation of scientists.
Visitors enjoyed chemical and pyrotechnic experiments, explored pond water under microscopes, solved quizzes, and tried puzzles or coloring pages. We greatly appreciate the positive feedback from children and parents.
"I am very glad we had the opportunity to present our work at the BIOCEV center to the local community. Scientific teams demonstrated chemical experiments, analyzed pond water, and prepared quizzes and coloring pages. The biggest joy for our scientists was the smiles on the children’s faces, who couldn’t get enough of the entertaining pyrotechnic experiments," shared Milan Jakubek, Head of Department.
We sincerely thank all colleagues who participated in the preparation and execution of the event.
Iva Hamová / Ondřej Havránek: Liquid biopsy for lymphoma treatment monitoring
Liquid biopsy is a new technique that allows to detect and evaluate tumor specific mutations within the pool of cell free DNA in peripheral blood, including lymphoma. It was shown that decrease or elimination of the tumor specific DNA from peripheral blood reflects lymphoma treatment effectivity, but this approach needs to be standardized and further evaluated in a real world setting. Team from Havranek laboratory, in collaboration with Department of Hematology of General University Hospital, showed that liquid biopsy is an excellent biomarker of treatment response which can clearly identify patiens that are not resending to standard therapy within a cohort of unselected patients that were treated by a standard chemoimmunotherapy. This is very important step towards liquid biopsy implementation into clinical practice and the goal for much needed further treatment personalization: de-escalation of treatment in good responders to limit acute and long term side effects and treatment intensification/change in patients predicted to relapse.
Vodicka P, Hamova I, Velasova A, Kupcova K, Zemankova P, Nehasil P, Tkachenko A, Lochovska K, Muzikova S, Hrabetova S, Dlouha J, Blahovcova P, Frouz T, Senavova J, Dlouha L, Polgarova K, Klanova M, Salkova J, Benesova K, Klener P, Trneny M, Havranek O. Circulating tumour DNA as a predictor of survival of patients with diffuse large B-cell lymphoma in a daily practice. Br J Haematol. 2025 Sep 7. doi: 10.1111/bjh.70128. (IF 3.8, Q1) [PubMed]


![]()
Andrea Gálisová from BIOCEV has been awarded a prestigious Starting Grant from the European Research Council
A major success: Charles University’s First Faculty of Medicine is receiving its first prestigious European Research Council (ERC) Starting Grant. It was awarded to Slovak biophysicist Andrea Gálisová, who, after completing her doctorate in Prague, also worked at the Weizmann Institute of Science in Rehovot, Israel, from 2018 to 2022. As an imaging methods expert, it was there that she came across her current research topic: extracellular vesicles, which until recently were considered a kind of “cellular waste.” What is it? Why are these particles interesting?
“Extracellular vesicles are small sacs made up of cell membranes that serve for intercellular communication. In our project, we will modify them using molecular biology methods so that they can deliver drugs, imaging agents, or membrane proteins to selected tissues and cells in a targeted manner. We will also monitor the modified vesicles in a living organism (mice) using the state-of-the-art heteronuclear magnetic resonance method,” explains Gálisová, describing the contents of her ERC project.
She currently works at the Institute for Clinical and Experimental Medicine (IKEM) in Prague’s Krč district, where implements imaging methods for visualization of extracellular vesicles in mouse models, and at the BIOCEV research centre in Vestec, which is a joint facility of Charles University and the Academy of Sciences. Gálisová is a member and associate scientist of Jiří Zahradník’s Laboratory of Protein Engineering. The great atmosphere at the workplace, as experienced by both at the Weizmann campus, is confirmed by the label on the door of one of the laboratories: among the many titled academics is a certain ‘Dr. Peter Venkman’ – yes, the scientific star of the hit comedy Ghostbusters (1984)! However, Gálisová’s research is no longer mere science fiction.
Read the full interview HERE
Source: Forum - Charles University Magazine



Interviews with PhD students in BIOCEV
Mgr. Lucía Csergeová - Laboratory of Molecular Oncology - READ HERE
Mgr. Dušan Nemes - Laboratory of Stem Cells Proteomics - READ HERE
Bailasan Haidar, MSc. - Laboratory of Protein Ingineering - READ HERE


Excursion Days in BIOCEV
The BIOCEV Centre recently welcomed more than 50 students from ZŠ Zdiměřice and ZŠ Klíček for two science excursions.
Thanks to the involvement of our researchers, students explored various labs and took part in hands-on activities such as pipetting, microscopy, and interactive demonstrations focused on cell biology, stem cells, chemical reactions, and mouse models used in biomedical research.
The program was prepared by experts from the Hematology laboratories and Medicinal Chemisty laboratory (1st Faculty of Medicine, Charles University), the Institute of Experimental Medicine (IEM CAS) and the Czech Centre for Phenogenomics (IMG CAS).
We sincerely thank all participating scientists for inspiring the next generation of researchers!



Congratulations to our new and continuing GA UK grant investigators
Congratulations to our students on their new projects, as well as the approval of seven ongoing projects in the 22nd round of the GA UK competition.
New projects:
Samuel Herceg - "Regulace odbourávání rozvětvených mastných kyselin a její role v metabolismu rakovinných buněk"
Dušan Němec - "Vliv inhibice antioxidační obrany leukemických buněk na kombinovanou terapii azacytidinu a venetoclaxu"


Milan Jakubek received the Award of the Governor of the Central Bohemia Region
Milan Jakubek, Head of the Laboratory of Medicinal Chemistry and Head of the 1st Faculty of Medicine at Charles University in BIOCEV, received the Governor’s Award of the Central Bohemian Region for 2024 in the Science and Research category on Tuesday, March 25, at the Kolín Theatre. The award recognizes individuals whose work, achievements, or contributions are extraordinary. Nominees are proposed by the public, and the winners are selected by a jury of regional representatives and experts.This year, the jury reviewed 46 valid nominations. More information HERE



Symposium "Sharing Experience in Biomedical Research"
The Czech-Israeli symposium entitled "Sharing Experience in Biomedical Research," organized by the First Faculty of Medicine, Charles University and The Hebrew University of Jerusalem, The Faculty of Medicine, Institute for Medical Research, has already taken place on March 10-11, 2025, at the BIOCEV center.
Thank you to all participants, and we look forward to future collaborations and events!



Journal Club & Data Meeting
These regular voluntary meetings will include both journal club seminars, which will alternate with data meetings. This activity is intended for doctoral/master's students at the 1st Faculty of Medicine in BIOCEV and will be held on Tuesdays from 9-10 a.m. in seminar room U2.020.
Opening Data Meeting: Tuesday, March 4th, 2025, at 9 a.m., U2.020
More information can be found HERE.
Postdoctoral Fellow in Hemato-Oncology
The Hematology Laboratories of the First Faculty of Medicine, Charles University at BIOCEV have an opening for a Postdoctoral Fellow in Hemato-Oncology.
For more information click HERE
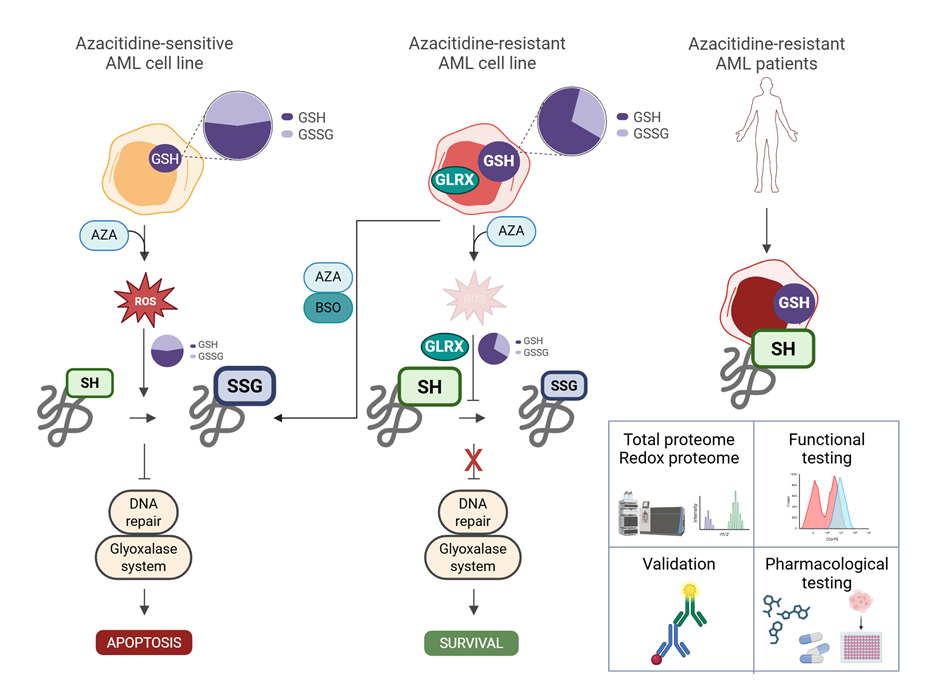
Jiří Petrák: New biomarkers of RVD in patients with heart failure
Běhounek M, Lipcseyová D, Vít O, Žáček P, Talacko P, Husková Z, Kikerlová S, Tykvartová T, Wohlfahrt P, Melenovský V, Beneš J, Petrák J. Biomarkers of RV Dysfunction in HFrEF Identified by Direct Tissue Proteomics: Extracellular Proteins Fibromodulin and Fibulin-5. Circ Heart Fail. 2025 Jan 17:e011984. doi: 10.1161/CIRCHEARTFAILURE.124.011984. (IF 7.9) [PubMed] JCR Q1

Congratulations to Dušan Němec
Dušan was awarded 7500 EUR with a travel grant from the European Hematology Association for his 6-month internship at Stem Cell Center, Lund University in Sweden.
https://ehaweb.org/research/grants/recipients/2024-winners/
Jiří Zahradník received a JUNIOR STAR grant for research on viral threats
The Czech Grant Agency will support nineteen JUNIOR STAR projects next year. One of them will also be implemented at the BIOCEV Centre, specifically in the laboratory of RNDr. Jiří Zahradník, Ph.D. from the 1st Faculty of Medicine of Charles University.

Viruses are an inseparable part of the world around us. For good and bad, they have influenced our history and will continue to shape our future. They have enriched us with biomolecules such as syncytin, a crucial protein player in the exchange of nutrients and gases between mother and fetus, essential for our very existence. However, what we more commonly associate with viruses is that they cause various unpleasant diseases.
In recent years, coronaviruses have gained considerable attention. These enveloped RNA viruses have characteristic spike proteins on their surfaces, which allow them to enter host cells. Coronaviruses exhibit an archetypal profile for cross-species transmission. They frequently spread from animal reservoirs and alter their genetic structure in response to new host environments.
“When we assume that human beings are the hosts, it is naturally expected that necessary changes will be similar even for human unrelated coronaviruses. Convergent evolution is a term we remember from biology classes, with dolphins and fish as an obvious example. It describes situations where similar adaptations emerge in unrelated groups of organisms independently of one another. This phenomenon isn’t just limited to aquatic vertebrates but encompasses a wide range of adaptations, including at the molecular level, and applies to viruses as well. Selected SARS-CoV-2 variants exhibited traits of convergent evolution, independently acquiring the same adaptive mutations,” explains Jiří Zahradník.
One of the goals of grant is to determine whether coronaviruses will undergo convergent changes when adapting to binding to human receptors and to identify factors that may lead to the emergence of viruses with a high affinity for human receptors.
“Based on mutagenic libraries, we plan to analyze which mutations increase the affinity of viral RBD domains to human receptors. The results could help us not only better understand past pandemics but also prepare for future pandemic threats, particularly regarding the prediction of new mutations and viral variants. Our research could also be applied to other respiratory viral pathogens,” comments Jiří Zahradník on his research.
You can read more about the supported projects HERE
Ondřej Havránek: Impact of PIK3CA gain and PTEN loss
Within a collaborative project with prof. Klener, this study from
Havranek laboratory described some of the key consequences associated
with phosphatidylinositol 3-kinase / protein kinase B (PI3K/AKT)
signaling pathway oncogenic activation. PI3K/AKT pathway is one of
central cellular pathways which is frequently altered by PIK3CA
amplifications and PTEN deletions in lymphomas. We have shown that in
mantel cell lymphoma, its oncogenic activation associates with lower
dependence on upstream activation, better metabolic and hypoxic
adaptation, and targeted therapy resistance. All features of more
aggressive tumor behavior with possible critical therapeutic
consequences.
Bettazova N, Senavova J, Kupcova K, Sovilj D, Rajmonova A, Andera L, Svobodova K, Berkova A, Zemanova Z, Daumova L, Herman V, Dolníkova A, Davis RE, Trneny M, Klener P, Havranek O. Impact of PIK3CA gain and PTEN loss on mantle cell lymphoma biology and sensitivity to targeted therapies. Blood Adv. 2024 Oct 22;8(20):5279-5289. doi: 10.1182/bloodadvances.2024013205. (IF 7.4, Q1) [PubMed]


Peter Dráber: TANK and AZI2 protect mice from TNF-induced cell death and autoinflammation
Ujevic A, Knizkova D, Synackova A, Pribikova M, Trivic T, Dalinskaya A, Drobek A, Niederlova V, Paprckova D, De Guia R, Kasparek P, Prochazka J, Labaj J, Fedosieieva O, Roeck BF, Mihola O, Trachtulec Z, Sedlacek R, Stepanek O, Draber P. TBK1-associated adapters TANK and AZI2 protect mice against TNF-induced cell death and severe autoinflammatory diseases. Nat Commun. 2024 Nov 19;15(1):10013. doi: 10.1038/s41467-024-54399-4. (IF 14.7) [PubMed] JCR D1


Ondřej Havránek: NOVEL CONCEPT - Vertical targeting of the PI3K/AKT pathway
Traditional cancer therapeutic combinations focus on targeting different signaling pathways and/or cellular processes at once. However, single agent-based targeting of a signaling pathway might not be sufficient for its inhibition. We have shown this for the PI3K/AKT pathway, where lymphoma cells normalized AKT activity within 24 hours even in the presence of a specific pathway inhibitors. To overcome this issue, we have developed a new approach that we called “vertical” signaling inhibition and showed that targeting PI3K/AKT pathway at multiple levels (multiple members) at the same time is synergistic, effective, and tolerated in vivo. Our results were just published in D1 journal!
Kupcova K, Senavova J, Jura F, Herman V, Rajmonova A, Pacheco-Blanco M,Chrbolkova T, Hamova I, Davis RE, Havranek O. Vertical targeting of the PI3K/AKT pathway at multiple points is synergistic and effective for non-Hodgkin lymphoma. Exp Hematol Oncol. 2024 Nov 1;13(1):108. doi: 10.1186/s40164-024-00568-6. (IF 9.4, Q1) [PubMed]


Jiří Petrák: Metabolomics and proteomics in pheochromocytoma and paraganglioma
Petrak J, Tevosian SG, Richter S, Ghayee HK. Metabolomics and proteomics in pheochromocytoma and paraganglioma: Translating biochemistry and biology to bedside. Best Pract Res Clin Endocrinol Metab. 2024 Sep 5:101935. doi: 10.1016/j.beem.2024.101935. (IF 6.1, Q1) [PubMed]


Hematopoietic stem cells use chromatin-remodeling factors for specific processes: the story of Smarca5
Mouse transgenic research suggests that lymphocyte development requires a robust chromatin remodeling. Turkova et al. found that although the chromatin-remodeling enzyme known as Smarca5 was thought to be a general factor, lymphocyte development in stem cells is most sensitive to its decrease.




The production of blood cells relies on coordinated regulation of gene expression, timing of the cell cycle, and suppression of inappropriate processes by apoptosis. Like any stem cell, the hematopoietic stem cell deals with the balance between stemness and differentiation, but also with the quantitative requirements for the production of different elements at specific stages of development and during everyday situations that might include bleeding or infections. The different decisions in the cell nucleus of stem cells are related to the structure of the DNA, which is wrapped in a bun of histone proteins. Different critical sequences have to be made available, and this is done by an enzymatic motor that releases DNA from the histone octamer, called the nucleosome, with the expenditure of energy, creating a region of freely accessible DNA, while other unnecessary DNA sequences are twisted like a thread around a spool. The unwinding of the DNA strand from the nucleosome is called the process of chromatin remodeling. It is generally accepted that stem cells have very little gene expression and replication, and thus we were interested in how dependent the various blood subtypes are on the function of the chromatin remodeling enzymatic engine in stem cells during their early differentiation. Specifically, we tested how the ISWI ATPase SMARCA5 (SNF2H), which represents an absolutely essential and unique enzyme, effects the production of lymphoid, erythroid and myeloid cells.
This type of project involves mouse transgenic models that we have gradually developed over the past 20 years. My lab group took up the project around 2005 in Prague, Czech Republic, but the roots go back to 1998 when we cloned the mouse mRNA for Smarca5 and found that Smarca5 is very important for blood production. In 2003, we published with Art Skoultchi (Albert Einstein College of Medicine, Bronx, NY) a mouse knockout for Smarca5 that showed its indispensability for early embryonic development. Further research led to the development of a mouse model of Smarca5 conditional inactivation that demonstrated the importance of Smarca5 for hematopoietic stem cell, lymphoid and erythroid development.
When we used up the possibilities of the knockout mouse and decided to create a conditional transgenic model for Smarca5. Although we created models for human as well as murine Smarca5, a publication now coming out in Communications Biology covers the first results with the transgene for human SMARCA5 entitled: Differential requirements for Smarca5 expression during hematopoietic stem cell commitment. We observed that the transgenic allele only provides about 10% of the expression of the normal allele. Therefore, we crossed the transgenic model with the previous model with a knockout allele to generate a set of supermodels in which the different Smarca5 expression levels exist. This allowed us to show that the hypomorphic (producing lower protein levels) SMARCA5 allele (S5tg) is fully functional and can rescue, in a dose-dependent manner, the gene inactivation phenotypes from previous k.o. projects, i.e. defects in lymphoid and erythroid development. In addition, transgenic Smarca5 binds all canonical complexes that have been incrementally discovered. Once we express low level of Smarca5 in stem cells, we see that progenitors entering lymphoid T and B development are the most sensitive to Smarca5 reduction, while other progenitors are less or not affected (see figure & legend).
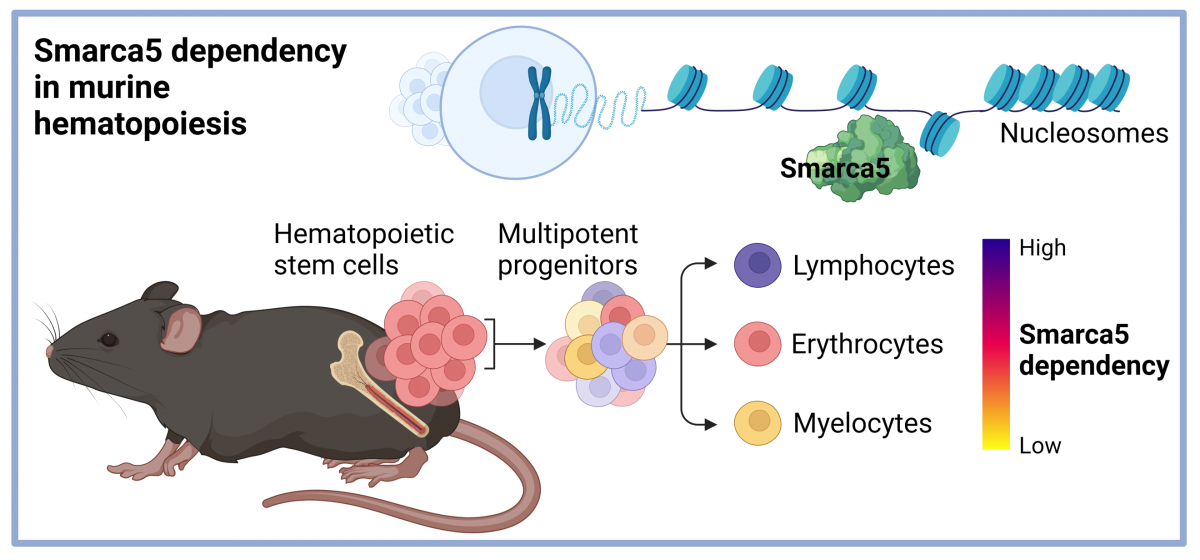
Figure legend: Hematopoietic stem cells in bone marrow regulate the expression of the epigenetic regulator Smarca5, which influences the development of blood elements through ATP-dependent effects on nucleosome architecture. Our data show that the development of lymphoid progenitors of both the B and T lineages is most dependent on Smarca5 levels, erythropoiesis is less dependent, while a very low level of the studied ISWI ATPase is sufficient for myeloid cell development. Created with BioRender.com.
To support our hypothesis that SMARCA5 expression level has a direct effect on development at stem cell level, we employed competitive transplantation experiment of transgenic and normal stem cells into normal but also transgenic recipients. Both of these experiments confirmed our hypothesis that it is primarily lymphocyte development that is strongly dependent on Smarca5 levels and that the defect originates in stem cells. Low SMARCA5 levels lead to significant permissiveness of the bone marrow niche for wild type stem cells. Transgenic hematopoietic stem cells were not able to produce lymphocytes, indicating that these lymphoid early progenitors are most sensitive to SMARCA5 levels. You think that's the end of the story? But no. Our current results show that Smarca5 is indeed a very important factor that affects the life of cancer stem cells. Therefore, we are also focusing on the question of how to effectively get rid of these cancer stem cells and one of the mechanisms is to focus on the biology of Smarca5 in this regard.
Authors: Tereza Turková a Tomáš Stopka
Publication: Turkova, T., Kokavec, J., Zikmund, T. et al. Differential requirements for Smarca5 expression during hematopoietic stem cell commitment. Commun Biol 7, 244 (2024). https://doi.org/10.1038/s42003-024-05917-z
Poslechněte si videokomentáře z letošní konference HEMATOLOGIE 2024
Co se událo na poli české a světové hematologie? ZDE

Cena kolegia děkana 1.LF UK za excelentní publikaci 2022
2. místo
Knizkova D, Pribikova M, Draberova H, Semberova T, Trivic T, Synackova A, Ujevic A, Stefanovic J, Drobek A, Huranova M, Niederlova V, Tsyklauri O, Neuwirth A, Tureckova J, Stepanek O, Draber P. CMTM4 is a subunit of the IL-17 receptor and mediates autoimmune pathology. Nat Immunol. 2022 Nov;23(11):1644-1652. doi: 10.1038/s41590-022-01325-9. (PubMed)
5. místo
Stopka T, Minařík L, Dusilková N, Pešta M, Kulvait V, Špaček M, Zemanová Z, Kalousová M, Jonášová A. G-CSF plus azacitidine versus azacitidine alone for patients with high-risk myelodysplastic syndrome: academic, open label, randomized trial. Blood Cancer J. 2022 Jul 7;12(7):105. doi: 10.1038/s41408-022-00698-2. (PubMed)
9. místo
Pimkova K, Jassinskaja M, Munita R, Ciesla M, Guzzi N, Cao Thi Ngoc P, Vajrychova M, Johansson E, Bellodi C, Hansson J. Quantitative analysis of redox proteome reveals oxidation-sensitive protein thiols acting in fundamental processes of developmental hematopoiesis. Redox Biol. 2022 Jul;53:102343. doi: 10.1016/j.redox.2022.102343. (PubMed)






Czech physicians and researchers to prolong the lives of people with leukaemia
A team of researchers and physicians from the BIOCEV Centre, 1st Department of Medicine - Hematology (the First Faculty of Medicine of Charles University) and the General University Hospital in Prague has designed a new approach for the treatment of acute leukaemia. This innovative method involves the combination of two medicines that, when administered together, have significantly prolonged the survival of patients. The clinical trial lasted five years and included 76 patients from the Czech Republic. Information about the new type of treatment was published by the prestigious Blood Cancer Journal from the Nature Publishing Group. At the moment, negotiations are underway to expand the trial to the European-wide level.
Doc. Anna Jonášová and Prof. Tomáš Stopka


Myelodysplastic syndrome (MDS) is a cancer of the bone marrow causing a low production of non-functioning blood cells. As a result, patients are deficient in both red and white blood cells, which are crucial for the body’s immunity, as well as platelets for blood coagulation. Patients suffering from this anaemia suffer from bruising, frequent internal bleeding and are less resistant to bacterial and viral infections, for example pneumonia.
Depending on the severity of the disease, antibiotics or repeated blood transfusions are given as treatment. In exceptional cases, patients can be treated with a bone marrow transplant. In Europe, four MDS patients per 100,000 people are diagnosed annually. In the Czech Republic, the General University Hospital registers approximately 80 patients each year. The critical phase of MDS is the transition to acute myeloid leukaemia (AML), characterised with a very short survival time.
"The current globally applied treatment for MDS patients during their transition to AML is based on 5-azacytidine, a medicine which was invented in the 1970s by the Czechoslovak Academy of Sciences. Azacytidine is an inhibitor of nuclear enzymes, referred to as DNA methylases, which are genetically and functionally disrupted in leukemic cells, and therefore, their activity helps to eliminate leukemic cells. Based on previous research, much of which has been published in reputable journals, our MDS/AML group has come up with the concept of “differentiation therapy for MDS”. We propose combining azacytidine with another substance called G-CSF, which promotes the formation and development of white blood cells in the bone marrow. We have been able to demonstrate the therapeutic effects of the new combination, and, above all, its safe effect on patients, but we have also been able to prolong their survival by months,” says Prof. Tomáš Stopka, head of research from the First Faculty of Medicine, Charles University, and Biocev. “At the same time, we have discovered that the combination of these agents induced significantly more treatment responses, which is ground-breaking and essential for further research and clinical testing in patients.”
A clinical trial demonstrating the benefit of the new treatment method in high-risk MDS was approved by the State Institute for Drug Control in 2017. The coordinator of the clinical trial was the Head of MDS and AML Treatment and Diagnosis, Doc. Anna Jonášová from the 1st Internal Clinic – Haematology, First Faculty of Medicine, Charles University in Prague: “We had to make a huge effort even before the trial itself was launched as we had to secure an application with the State Institute for Drug Control and have the trial pass through the ethics committees. The proposed therapeutic combination advances the use of the cytokine G-CSF in AML therapy, while its synergistic activity with azacytidine makes this treatment more effective and safer for patients, representing a proven strategy that is immediately applicable in practice. We would not have achieved the results if it had not been for the great willingness of the patients who participated in the trial."
At the moment, intensive negotiations are underway for a new European-wide trial in which another medicine, Venetoclax, will be investigated alongside 5-azacytidine and G-CSF. "I am happy that we are successfully linking genetic and clinical data in the Czech Republic and thanks to this, we are able to determine how acute leukaemia will be treated in the near future," adds Prof. Tomáš Stopka.
Link to the publication: HERE
Rozhovor pro UK FORUM: Nová kombinace léčiv prodlužuje život pacientů s leukémií (ukforum.cz)